In-vivo microinjection technique enables us to work on a specific neuronal population in central nervous system.
We use state of art techniques to deposit specific compounds in very low volumes (50-100 nL) onto the targeted neuronal sites in rodent brain. Our technique involves with pressure-based or electrically-operated microinjections through pulled glass capillaries.
We inject compounds including drugs, viral vectors, and tracer molecules that enable us to examine the neuronal projections of identified cells in slices.

Figure.1 Our pressure-based microinjection setup includes 1: Large animal stereotaxic frame, 2: Stereomicroscope, 3: Metal-halide light source, and 4:Pressure-based microinjection system (Picospritzer).
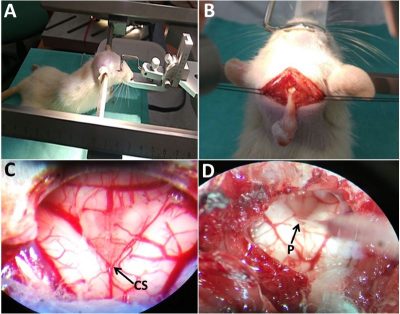
Figure.2 represents a performed brainstem microinjection application in a rat. A: rat is fixed in a large animal frame, B: caudal medulla is exposed through a blunt dissection, C: closer view of the medullary surface and the base of the fourth ventricle, and D: a glass micropipette that is placed in left dorsal vagal complex. CS: calamus scriptorius, P: micropipette.
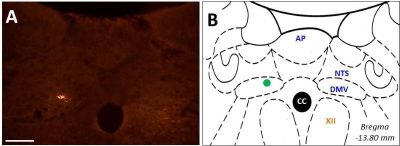
Figure.3 Post-mortem histological verification of an injection site. A: detection of fluorospheres in dorsal motor nucleus of N.vagus. B: green dot depicts the injection site in the schematic. CC: canalis centralis, NTS: solitary tract, DMV: dorsal motor nucleus of N.vagus, AP: area postrema.
Son güncelleme : 9.10.2023 00:46:51
